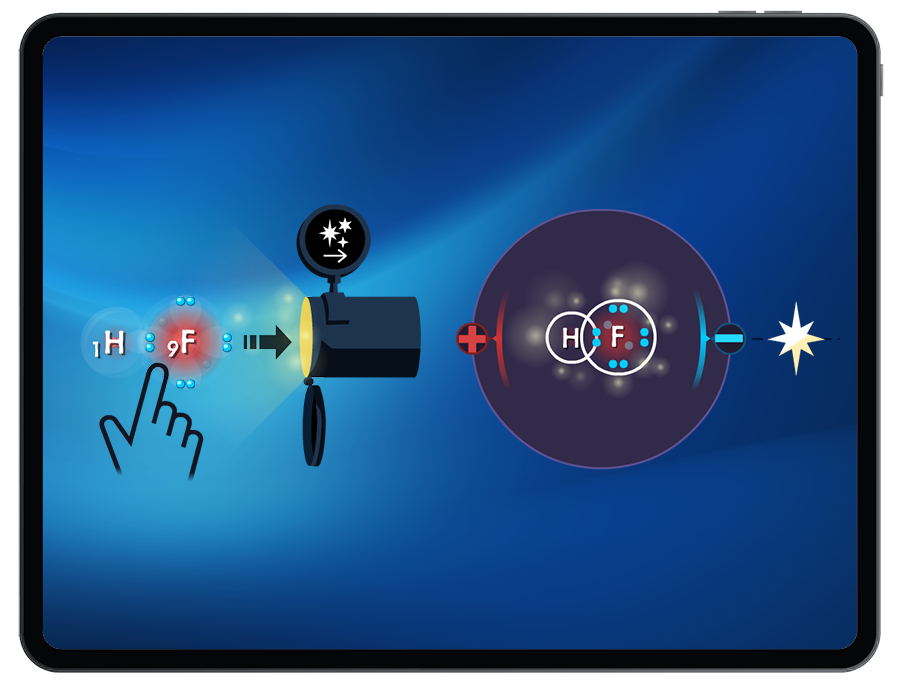
Intermolecular Forces
Use single atoms or build molecules of target polarities to create intermolecular forces.
Topics
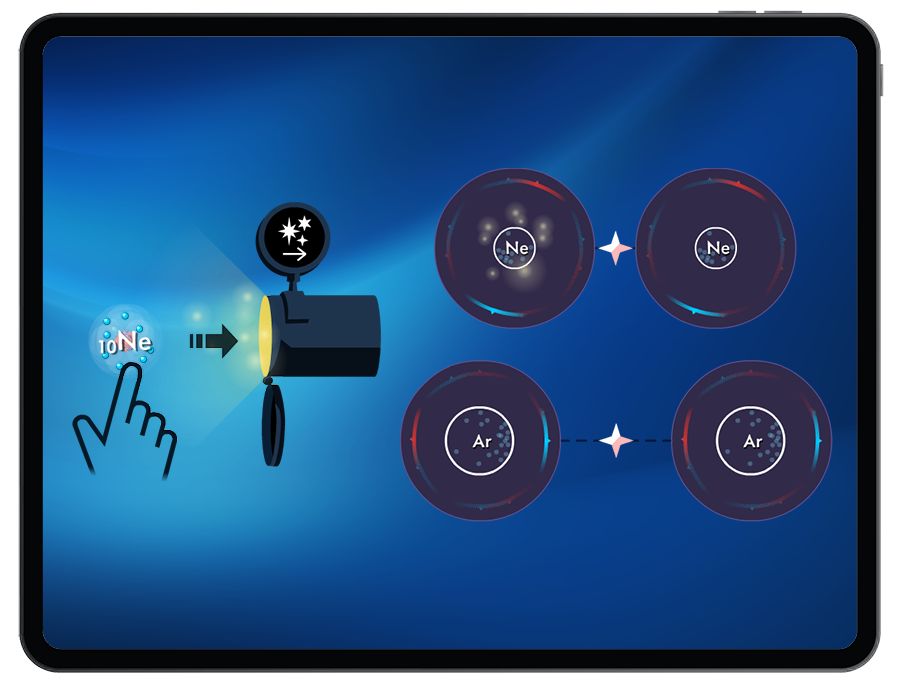
- London Dispersion Forces
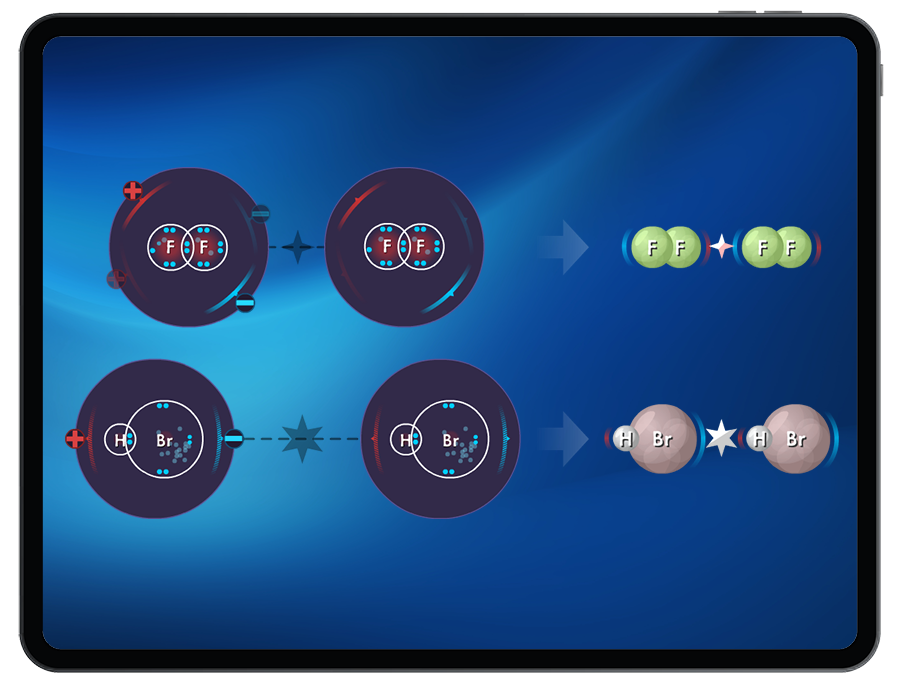
- Dipole-dipole interactions
- Hydrogen bonding
- Relative IMF strengths
- Polar and nonpolar bonds
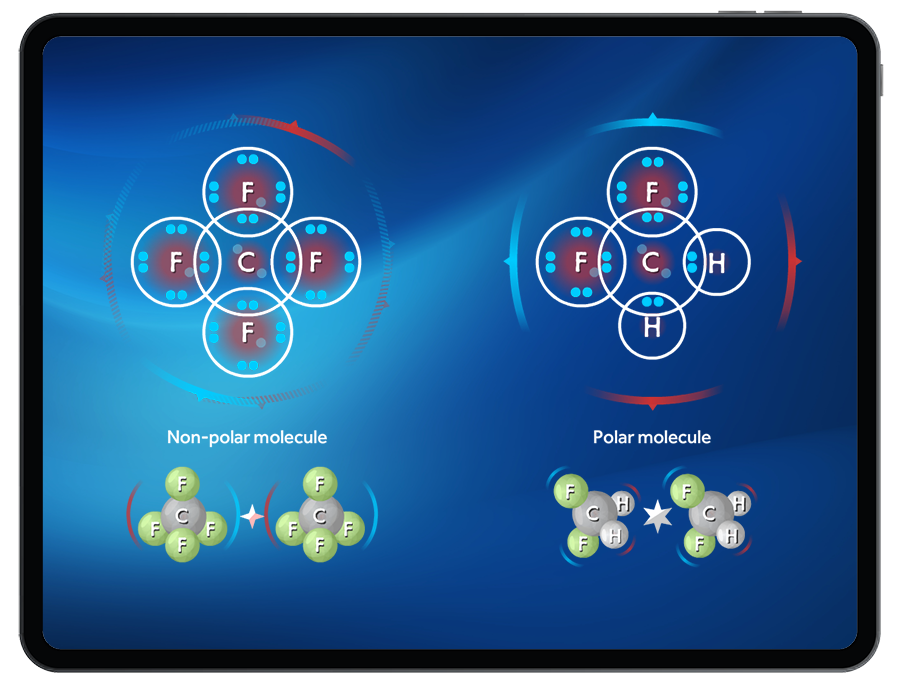
- Polar and nonpolar molecules
- Molecular geometry and polarity
Core levels
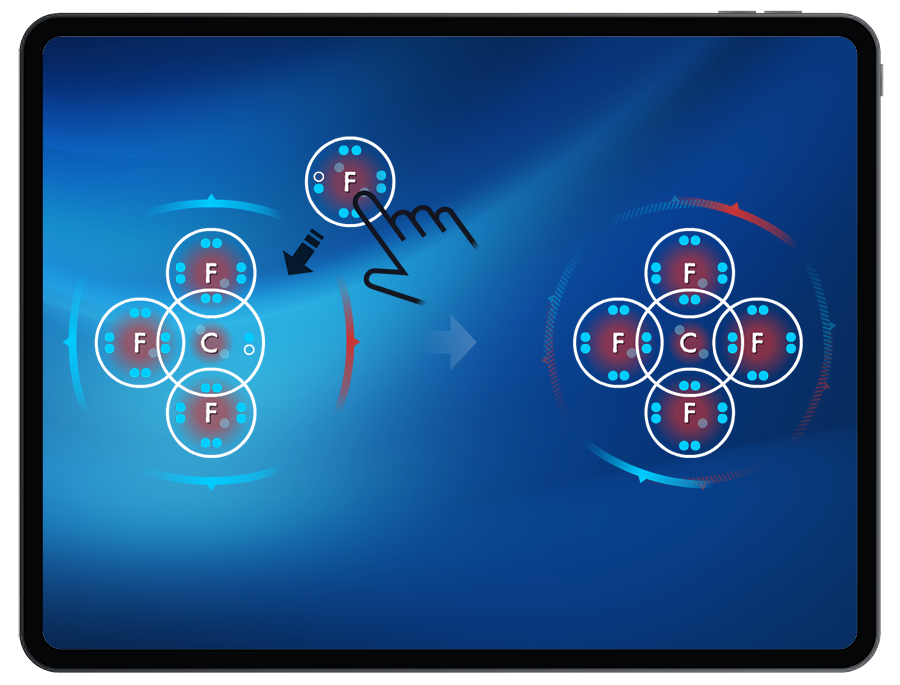
Students receive live polarity feedback as they build polar and nonpolar molecules.
Students explore how molecular polarity can influence the type of intermolecular force (London Dispersion Forces, dipole-dipole interactions, and hydrogen bonding) that forms between atoms or molecules.
Sandbox
The IMFs Sandbox is an open-ended and exploratory environment designed for students to freely explore molecular polarity and intermolecular forces. Complement your instruction by designing your own Sandbox activities and encourage your students to earn the built-in Achievements that focus on a specific topic within Intermolecular Forces.
Connected levels
Lewis Structures and IMFs
In these connected levels, there are molecules missing from the bank. Students must return to the Lewis Structures game to build the missing molecules of specific polarity that they need in order to form the correct IMFs in the Intermolecular Forces game.
Radii Trends and IMFs
In these connected levels, there are atoms missing from the bank. Students must return to the Radii Trends game to build the specific atoms that they need in order to create the target IMFs in the Intermolecular Forces game.